This biotech CEO decided to take her own (fertility) medicine

For either group, a simplified process could be attractive. “Convenience. That is what’s interesting,” says De Vos.
That’s also one reason Radenkovic is betting that Gameto’s technology will be “influential” in the growing egg freezing market. “As a woman, when you’re undergoing IVF, you want a baby then. So you have a stronger desire to go through a difficult process. And often, not always, you have a partner who’s helping both financially and emotionally. So you’re kind of going to put up with it,” she says. “Whereas if you’re egg freezing … it’s to keep options open.”
Woolly mammoths
The company’s technology was initially developed in a Harvard University laboratory led by the geneticist George Church. Researchers there had been devising methods of quickly turning stem cells into any other cell type, often in just a few days. The trick was to add extra genes that, when turned on, would impose a developmental program on the cells, causing them to become, say, nerve or heart cells.
Church and his students were particularly interested in making eggs. If human eggs could be directly constructed in the lab, it would theoretically allow researchers to make them for all patients, no matter their age—basically solving the problem of ovarian age. Equally important to Church was a subplot then unfolding in his lab, in which a student had begun introducing woolly mammoth genes into elephant cells. He wanted to re-create the extinct pachyderm, but to do that, the project would need potentially thousands of elephant eggs. And the only way to get them would be to manufacture them.

CHRISTIAN KRAMME/GAMETO
But making mammalian eggs has turned out to be a hard problem. It’s been done in mice, but not yet in any other species. (“We’re still trying,” Church told me.) Part of the difficulty is the sheer size of eggs, which are about 8,000 times bigger than a white blood cell. Instead, by 2022, the lab was finding success using stem cells to manufacture other components of the ovary, in particular granulosa cells—tissues in follicles that emit estradiol and play a key role in sending maturation signals to the egg.
That turned out to be the technology Gameto needed to mature eggs in a dish, and so the company licensed patent rights from Harvard and also hired one of Church’s students, Christian Kramme, to lead its science efforts and become its vice president of cell engineering. (Patent rights involving elephants, kangaroos, and other nonhuman mammals went to a different startup, Colossal Biosciences, which intends to re-create several extinct species.)
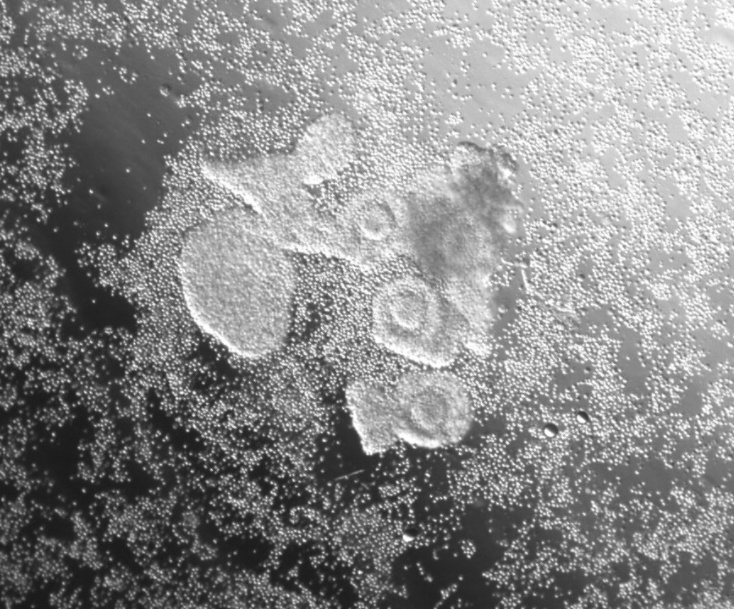
Radenkovic says the company’s product, which it calls Fertilo, will essentially be a tube of frozen granulosa cells that can be sprinkled around an egg to help it develop. In the paper published last week in Human Reproduction, they reported that adding these cells to a petri dish had significant positive effects on eggs, causing more of them to mature successfully. Photographs of the process show egg complexes (so called because they are still wrapped in protective tissue) with the granulosa cells appearing around them like small punctuation marks. Although the details of how it works aren’t entirely clear, it appears that molecular cross talk between these supporting cells and eggs helps them finish their maturation in an organized manner.
Baby in the works
In Gameto’s study, some of the eggs collected were also fertilized with sperm from a donor bank to test their potential to make embryos. Because some of those eggs belonged to Radenkovic, I asked if she had any personal feelings toward the embryos. While only balls of a few hundred cells, they were, technically, her offspring, and they were later destroyed. Radenkovic didn’t answer my question directly, but she agreed there was a weighty issue here. She said it was about managing possible harms and benefits. The company absolutely needed to demonstrate the embryos were normal, according to a battery of tests. Without that information, it would not be able to proceed to the next step: making a baby. At the same time, she says, they made as few embryos as they could. That part of the experiment was stopped as soon as the data collected cleared the bar of statistical significance.




