Stanford scientists figured out why lithium metal batteries fail
Researchers at Stanford University and the US Department of Energy’s SLAC National Accelerator Laboratory have identified what causes lithium metal batteries to short-circuit and fail – and this could help avoid the problem in future battery production.
The post Stanford scientists figured out why lithium metal batteries fail appeared first on Electrek.
Researchers at Stanford University and the US Department of Energy’s SLAC National Accelerator Laboratory have identified what causes lithium metal batteries to short-circuit and fail – and this could help avoid the problem in future battery production.
As a result of this discovery, energy-dense, fast-charging, nonflammable lithium metal batteries that last a long time could overcome the main barriers to their use in EVs, among other benefits.
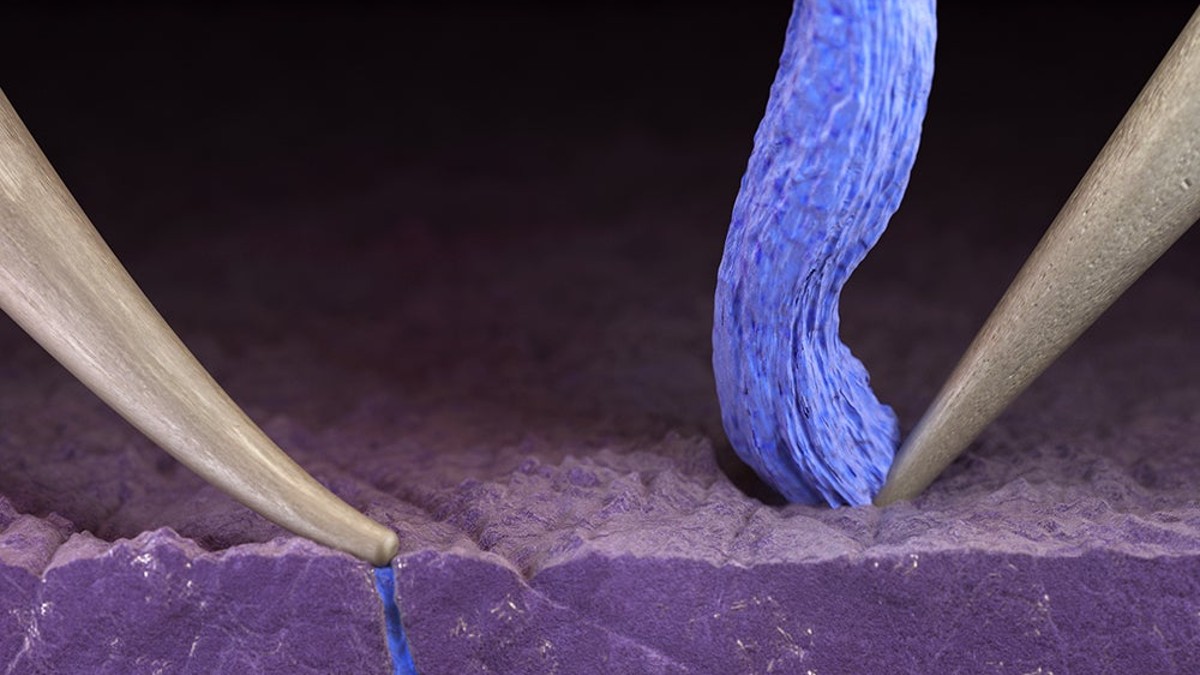
Lithium metal batteries with solid electrolytes are lightweight, inflammable, pack a lot of energy, and can be recharged very quickly. There’s just been a short-circuiting problem that causes them to fail.
But researchers appear to have pinpointed the problem. In a paper published in the journal Nature Energy, titled “Mechanical regulation of lithium intrusion probability in garnet solid electrolytes,” researchers cited mechanical stress, especially during potent recharging, as the cause of failure.
Senior author William Chueh explains:
Just modest indentation, bending or twisting of the batteries can cause nanoscopic fissures in the materials to open and lithium to intrude into the solid electrolyte, causing it to short circuit.
Even dust or other impurities introduced in manufacturing can generate enough stress to cause failure.
Colead author Xin Xu likened it to the way a pothole appears in pavement. Through rain and snow, car tires pound water into the tiny, preexisting imperfections in the pavement, producing ever-widening cracks that grow over time.
Xu said:
Lithium is actually a soft material, but, like the water in the pothole analogy, all it takes is pressure to widen the gap and cause a failure.
So the researchers are now looking at ways to use these very same mechanical forces to toughen the material during manufacturing, much like a blacksmith anneals a blade during production. They’re also looking at ways to coat the electrolyte surface to prevent cracks or repair them if they emerge.
Scientists around the world working to develop new solid electrolyte rechargeable batteries can design around the problem, or even turn the discovery to their advantage, as scientists at Stanford are now researching.
Main image section: Cube3D
Read more: Porsche to design 3D-printed battery gigafactories for Sakuu
UnderstandSolar is a free service that links you to top-rated solar installers in your region for personalized solar estimates. Tesla now offers price matching, so it’s important to shop for the best quotes. Click here to learn more and get your quotes. — *ad.
FTC: We use income earning auto affiliate links. More.




