The effort to make a breakthrough cancer therapy cheaper
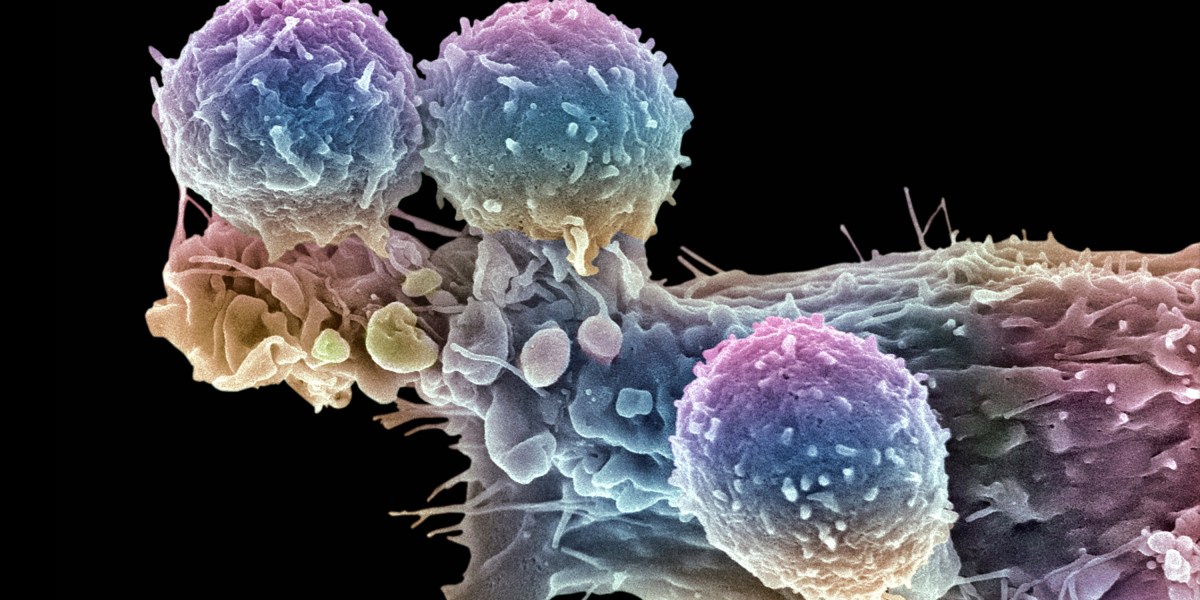
It’s an attractive model. Demand for CAR-T often outstrips supply, leading to long wait times. “There is a growing tension around the limited access that we’re seeing for cell and gene therapies coming out of biotech,” Stanford pediatric oncologist Crystal Mackall told Stat. “It’s incredibly tempting to say, ‘Well, why don’t you just let me make it for my patients?’”
Even these treatments run in the tens of thousands of dollars, partly because approved CAR-T products are bespoke therapies, each one produced for a particular patient. But many companies are also working on off-the-shelf CAR-T therapies. In some cases, that means engineering T cells from healthy donors. Some of those therapies are already in clinical trials.
In other cases, companies are working to engineer cells inside the body. That process should make it much, much simpler and cheaper to deliver CAR-T. With conventional CAR-T therapies, patients have to undergo chemotherapy to destroy their existing T cells. But with in vivo CAR-T, this step isn’t necessary. And because these therapies don’t require any cell manipulation outside the patient’s body, “you could take it in an outpatient clinic,” says Priya Karmali, chief technology officer at Capstan Therapeutics, which is developing in vivo CAR-T therapies. “You wouldn’t need specialized centers.”
Some in vivo strategies, just like the ex vivo strategies, rely on viral vectors. Umoja Biopharma’s platform uses a viral vector but also employs a second technology to prompt the engineered cells to survive and expand in the presence of the drug rapamycin. Last fall, the company reported that it had successfully generated in vivo CAR-T cells in nonhuman primates.
At Capstan Therapeutics, researchers are taking a different tack, using lipid nanoparticles to ferry mRNA into T cells. When a viral vector places the CAR gene into a cell’s DNA, the change is permanent. But with mRNA, the CAR operates for only a limited time. “Once the war is over, you don’t want the soldiers lurking around forever,” Karmali says.
And with CAR-T, there are plenty of potential battlefields to conquer. CAR-T therapies are already showing promise beyond blood cancers. Earlier this year, researchers reported stunning results in 15 patients with lupus and other autoimmune diseases. CAR-T is also being tested as a treatment for solid tumors, heart disease, aging, HIV infection, and more. As the number of people eligible for CAR-T therapies increases, so will the pressure to reduce the cost.
Read more from MIT Technology Review’s archive
Scientists are finally making headway in moving CAR-T into solid tumors. Last fall I wrote about the barriers and the progress.
In the early days of CAR-T, Emily Mullin reported on patient deaths that called the safety of the treatment into question.




